Abstract
Background There has been no prospective previously applied clinical prognostic score in pediatric Hodgkin lymphoma (HL). The CHIPS (Childhood Hodgkin International Prognostic Score), a predictive model for Event Free Survival (EFS), was developed in training and validation cohorts of patients with intermediate risk HL treated with 4 cycles of chemotherapy plus radiation therapy on AHOD0031 (NCT00025259).1
Prospective validation of CHIPS for prediction of EFS in high-risk HL was a pre-specified secondary aim of AHOD1331, a trial comparing the efficacy of Brentuximab (Bv) with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide (Bv-AVE-PC) to the standard pediatric dose intensive regimen ABVE-PC, inclusive of bleomycin with response adapted radiation therapy. For this analysis, our primary goal was to assess the validity of CHIPS in the whole population of patients with high-risk disease.
Methods AHOD1331 was a multicenter randomized, open-label phase 3 study that enrolled patients 2-21 years with previously untreated HL, stages IIB + bulk, IIIB, IVA, IVB (NCT02166463). Score components of CHIPS (Stage 4 disease, large mediastinal mass, albumin (<3.5), fever) were collected prospectively. Scores range from 0-4 determined by assigning 1 point for each component. CHIPs was evaluated as a predictor of EFS and of early response by PET after 2 cycles of therapy (iPET).
Events for EFS included disease relapse/progression, second malignancy, and death due to any cause. Time-to-event was computed from study enrollment; patients without failures were censored at date of last contact. Multivariable Cox Regression was used to examine the predictive value of CHIPS.
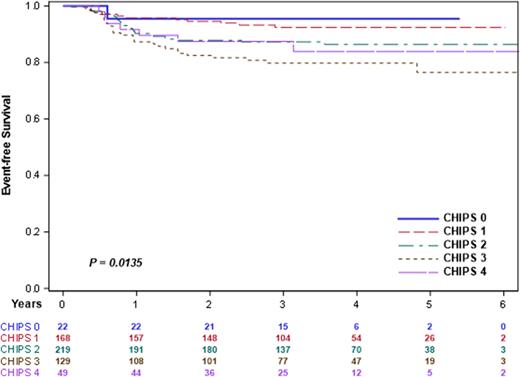
Results The distribution of CHIPS among 587 high risk patients eligible for AHOD1331 did not differ by study treatment arm (p=0.2405). 3-yr EFS differed overall by CHIPS score in this high-risk cohort (Figure 1, p=0.0135). The trend for CHIPS predictive potential was similar by treatment arm, and the comparison EFS for CHIPS 0,1 vs CHIPS 2,3,4 was statistically significant for each treatment arm (Bv-AVE-PC, p= 0.0333; ABVE-PC p= 0.0402). In multivariate model including treatment arm, CHIPS was an independent predictor of EFS (p=0.0203). CHIPS was also predictive of interim response (iPET, p=0.0173) at interim PET.
Conclusion CHIPS is a baseline clinical score that is predictive of interim response and EFS among pediatric high-risk HL patient and was shown to be agnostic to treatment arm in this study. Based on clinical factors known at the time of diagnosis, CHIPS may aid in the allocation of patients to risk-based treatment algorithms.
1Schwartz CL, et. al, Pediatr Blood Cancer. 2017 Apr;64(4).
Figure 1: EFS by CHIPS
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal